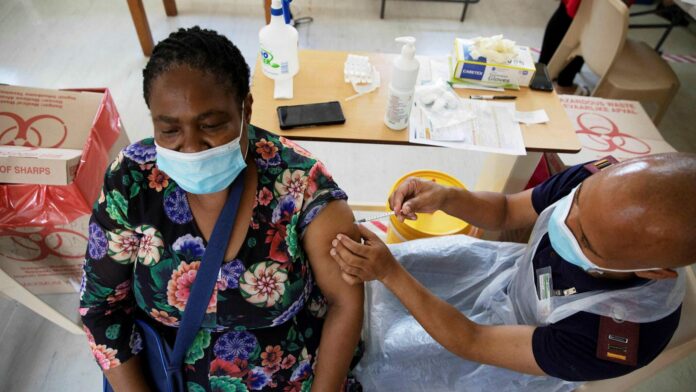
South Africa, which is late in the Corona vaccination campaign and has just entered the third wave of the epidemic, announced that it has withdrawn two million vaccines from Johnson & Johnson, due to a “non-conformity problem” when manufactured in the United States.
The South African Medicines Agency (Sahpra) said in a statement that it had “taken a decision not to distribute vaccines produced from batches of unsuitable pharmaceutical ingredients.”
And the US authorities announced that “several batches”, that is, several million doses, that were manufactured in Baltimore in the United States, and whose production stopped several weeks ago, will have to be disposed of.
mixing doses
Tests revealed that ingredients in the British AstraZeneca vaccine, made in the same factory, had been mistakenly mixed into Johnson & Johnson’s formula.
And the South African Minister of Health, Mamoloko Kobay-Ngoban, had said that his country had known a “step back in the vaccination programme”, he said that the batches in question are those that are currently stored in a high-tech laboratory in Port Elizabeth (south of the country).
The Aspen Laboratory in South Africa imports Johnson & Johnson vaccine components from this site and packages them on site.
South Africa is counting on providing 31 million doses of the single-dose Johnson & Johnson vaccine to immunize its 59 million people.
The country managed to obtain 30 million doses of the Pfizer vaccine, but this vaccine needs to be stored at very low temperatures.
Only 1 percent of the population is vaccinated
South African officials said a new shipment of 300,000 “approved” vaccines from Johnson & Johnson arrived on Tuesday.
The government had already temporarily suspended the vaccine in April, after cases of blood clots in the United States. In February, it also gave up 1.5 million doses of AstraZeneca, after doubts about its efficacy against the local mutated virus “Beta”.